Graham Lab News
Events
2024 Alzheimer\'s Association International Conference
Brain glutathione in vascular mild cognitive impairment and cognitive correlates
Methods: Possible vMCI patients (1 standard deviation (SD) below population norms in verbal memory, executive function (EF), processing speed, or working memory, age 55-85, and currently enrolled in a 6-month exercise rehabilitation program due to having 2 or more vascular risk factors or previous vascular event) and cognitively-normal (CN) controls were recruited. All participants received 1H magnetic resonance spectroscopy (MEscher–GArwood Point Resolved Spectroscopy) to quantify brain GSH at baseline in the anterior cingulate (AC) and occipital lobe (OL). Spectroscopic analysis was completed using the Gannet toolkit (vers. 3.1) in Matlab (vers. 2020b).
Results/Conclusions: In 43 participants (mVCI n = 22, CN n = 21), AC-GSH (I.U. ± SD) was higher in mVCI (1.96 ± 0.29) compared to CN (1.64 ± 0.48) (F(1,29.7) = 6.9, p = .01); this difference remained after correcting for cerebrospinal fluid (CSF) volume (F(1,28.4) = 6.5, p = .02), and controlling for age and sex (B [SE] = 0.33 [0.11], p = .007). There was no difference in OL-GSH before or after correcting for CSF volume. Higher AC-GSH, but not OL-GSH, was correlated with poorer global cognition (Montreal Cognitive Assessment -Full score, B [SE] = 2.15, p = .03), and poorer EF performance (B [SE] = -0.67 [0.25], p < .001). These relationships remained significant after correcting for CSF volume.
The current study suggests an upregulation of AC glutathione in vMCI, which may reflect a compensatory increase in antioxidants as a response to oxidative stress challenges reported in these patients. Higher brain GSH in the AC region is correlated with poorer global cognition and executive function performance, suggesting a link between local brain antioxidant response and disease-relevant cognitive domains.
*J. J. CHEN1,2, N. HERRMANN1,3,4, K. SURVILLA5, E. MAH1,2,4, Y. KANG1, S. E. BLACK1,6,7,8, J. RAMIREZ8,9, A. C. ANDREAZZA10, A. KISS11,12, P. I. OH13, D. GALLAGHER1,2,8, S. J. GRAHAM4,14, K. L. lANCTÔT1,2,13,15,16.
Sunday, July 28, 2024 at 8:00 AM-11:55 PM
A pilot study evaluating the feasibility, safety, and efficacy of transcranial photobiomodulation (tPBM) for the treatment of mild cognitive impairment (MCI): preliminary findings
Lipid peroxidation in patients with vascular mild cognitive impairment from a memory and antioxidants in vascular impairment trial (MOVE-IT)
1University of Toronto, Toronto, ON, Canada, 2Sunnybrook Research Institute, Toronto, ON, Canada, 3Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 4Center for Addiction and Mental Health, Toronto, ON, Canada, 5KITE Research Institute, Toronto Rehabilitation Institute, Toronto, ON, Canada, 6University of Toronto, Torono, ON, Canada.
2023 Society for Neuroscience Annual Conference
Dr. Graham and members of the Graham Lab attended the Society for Neuroscience\'s Annual Conference in Washington, D.C. You can explore the conference abstracts they contributed in the following tabs.
Brain Effects of Post-COVID-19 Condition Observed by Dynamic Susceptibility Contrast Magnetic Resonance Imaging
Methods: To date, DSC data have been collected for 14 healthy controls (9 female (F), mean (standard deviation) age = 44(13)), and 63 PCC participants (55 self-isolated while infected, 37 F, age = 42(12); 8 hospitalized while infected, 5 F, age=54(11)). DSC MRI was performed at 3 T (1.74 mm in-plane resolution, 8 mm slices, 140 time points, 1.25 s temporal resolution) using Gadovist contrast agent. Data were analyzed with a custom pipeline to generate CBF and K2 maps (see representative healthy control data, Fig. 1) followed by voxel-wise two-tailed t-tests with correction for multiple comparisons.
Results/Conclusions: No statistically significant group differences were observed, but there were notable trends between self-isolated PCC individuals and controls for CBF and K2 when a region of interest analysis was conducted for the thalamus - consistent with previous NeuroCOVID19 CBF results obtained using arterial spin labeling[3], and with reports that leaky brain vasculature elevates risk of acute COVID19[1]. These results help confirm that there are observable changes in cerebrovascular physiological parameters due to PCC, either as a direct or indirect consequence of SARS-CoV2 infection. 1. Macintosh BJ et al., CMAJ Open 2021.2. Boxerman JL et al., AJNR 2006.3. Kim WSH et al., JMRI 2022.
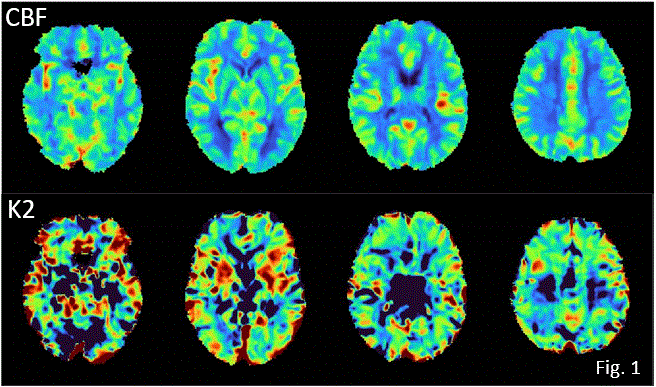
*S. J. GRAHAM1, A. PAVEL1, F. O\'HARA1, N. W. CHURCHILL3, F. TAM1, F. GAO2, M. MASELLIS2, B. LAM2, I. CHENG2, C. HEYN2, E. ROUDAIA4, J. CHEN4, S. E. BLACK2, A. B. SEKULER4, T. A. SCHWEIZER3, B. MACINTOSH2
1Physical Sciences, Sunnybrook Res. Inst., 2Hurvitz Brain Sci. Program, Sunnybrook Res. Inst., Sunnybrook Hlth. Sci. Ctr., Toronto, ON, Canada; 3St. Michael\'s Hospital, Unity Hlth. Syst., Toronto, ON, Canada; 4Rotman Res. Inst., Baycrest, Toronto, ON, Canada
Sunday, November 12, 2023 at 1:00 PM-5:00 PM
Post-covid fatigue is associated with abnormal subcortical texture in T1-weighted MRI
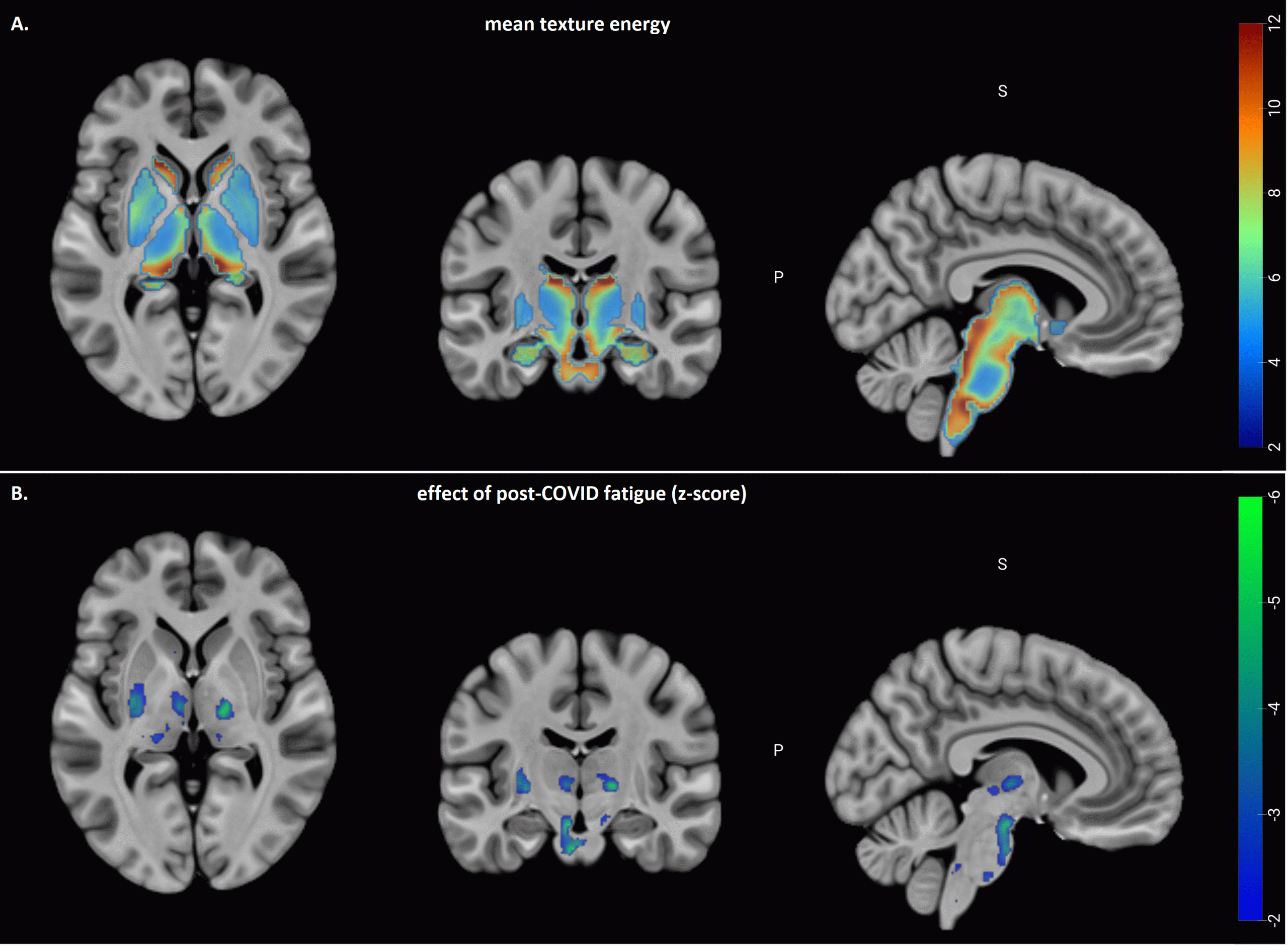
*N. CHURCHILL1, E. ROUDAIA2, J. J. CHEN3, A. B. SEKULER3, F. GAO4, M. MASELLIS5, B. LAM4, I. CHENG4, C. HEYN4, S. E. BLACK6, B. J. MACINTOSH4, S. J. GRAHAM7, T. A. SCHWEIZER8
1St. Michael\'s Hosp., Toronto, ON, Canada; 2Rotman Res. Inst., Montreal, QC, Canada; 3Baycrest Hosp., Toronto, ON, Canada; 4Sunnybrook Hosp., Toronto, ON, Canada; 5Sunnybrook Res. Inst., Toronto, ON, Canada; 6Dept Med. (Neurol), Sunnybrook Hlth. Sci. Cntr, Toronto, ON, Canada; 7Research, Physical Sci., Sunnybrook Hlth. Sci. Ctr., Toronto, ON, Canada; 8Univ. of Toronto, Toronto, ON, Canada
Measures of cortical thickness in Alzheimer\'s disease with and without history of traumatic brain injury
Methods: The present study examined this issue using a mixed design approach, applied to a cohort of 1124 participants from the National Alzheimer’s Coordinating Center (NACC) including 343 with AD, 127 with AD with TBI, 266 cognitively normal adults with TBI, and 388 cognitively normal adults without TBI. For these groups, cortical thickness measures were obtained from T1-weighted magnetic resonance imaging (MRI) data using FreeSurfer and in-house software. An initial cross-sectional analysis of this group at baseline used multiple linear regression to determine the interaction effects of AD and TBI on measures of cortical thickness. Among those with AD, TBI was associated with an earlier age of AD onset but, counter-intuitively, less cortical thinning in fronto-temporal regions, relative to non-AD controls.
Results/Conclusion: The results suggest that AD with TBI represents a physiologically distinct group from AD at baseline assessment. A second longitudinal analysis of this group used a partial least squares approach to measure group differences in the longitudinal change of cortical thickness values, for a subset of 154 participants with follow-up scans, assessed over an average time span of 33 months. The AD groups with and without TBI history more strongly expressed patterns of longitudinal frontal and temporal atrophy, while the cognitively normal control group displayed an intermediate pattern of atrophy, and the cognitively normal TBI group expressed the least atrophy.
Further, comparison of AD and AD+TBI to their respective control groups showed a more pronounced effect of AD for the TBI groups. These results provide preliminary evidence of a relationship between TBI history and risk of accelerated cortical thinning in frontal and temporal regions. An improved understanding of the long-term outcomes of TBI has the potential to aid in the diagnosis and treatment of individuals with AD combined with history of TBI.
*G. M. D\'SOUZA1,2, , N.W. CHURCHILL2,3, D. X. GUAN4, M. A. KHOURY1,2, S. J. GRAHAM1,5, S. KUMAR1,6, C. E. FISCHER1,2, T. A. SCHWEIZER1,2 1Univ. of Toronto, Toronto, ON, Canada; 2St. Michael\'s Hosp., Toronto, ON, Canada; 3Toronto Metropolitan Univ., Toronto, ON, Canada; 4Univ. of Calgary, Calgary, AB, Canada; 5Sunnybrook Res. Inst., Toronto, ON, Canada; 6Ctr. for Addiction and Mental Hlth., Toronto, ON, Canada
Wednesday, November 15, 2023 at 8:00AM-12:00PM
Post-concussion changes in functional network connectivity relative to the pre-injury brain
Methods: In this study, a large sample of 167 varsity athletes had resting-state functional magnetic resonance imaging (fMRI) collected at pre-season baseline. Of this cohort, 25 were later concussed, with imaging at acute injury, medical clearance, and up to one year later. An additional 27 athletes without concussion were re-imaged as controls.
Results/Conclusion: Concussed athletes showed significant post-concussion declines in anterior brain connectivity lasting beyond medical clearance. This study provides the first characterization of brain function after concussion, relative to the pre-injury brain. The results of this study indicate that disturbances in connectivity are present at and beyond medical clearance, highlighting the complex, long-term nature of recovery after injury.
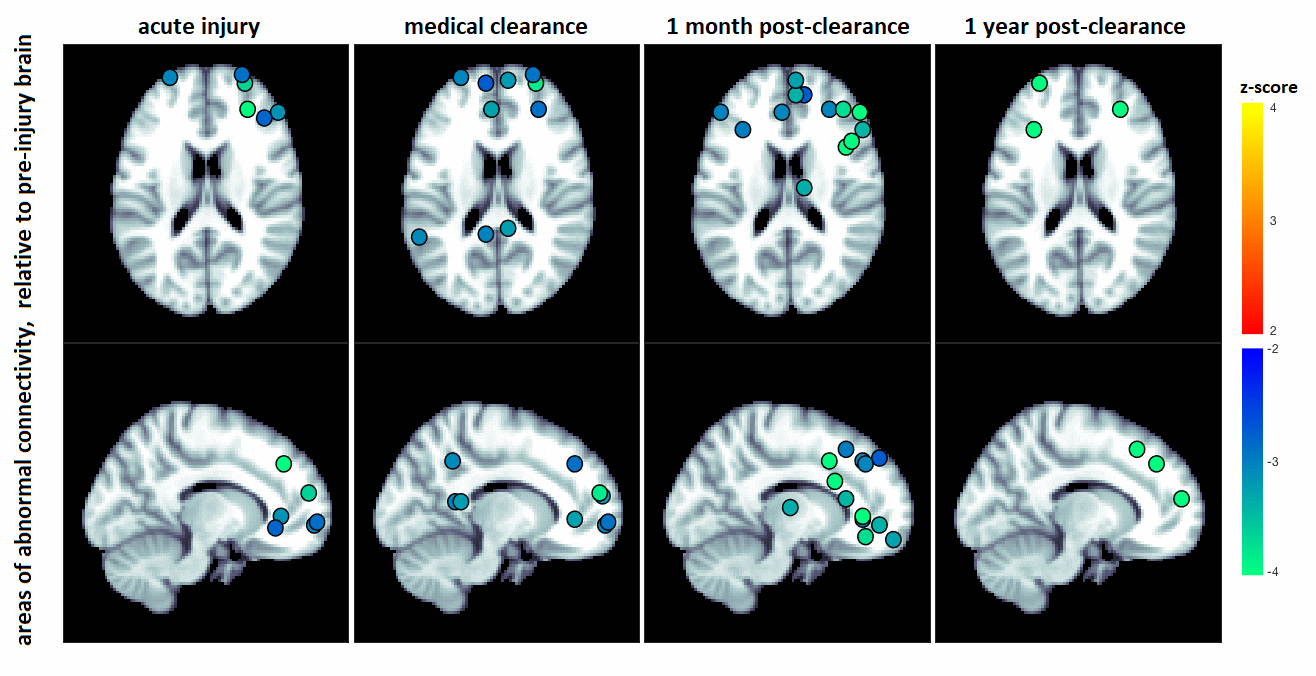
N. CHURCHILL1, M. HUTCHISON2, S. J. GRAHAM3, *T. SCHWEIZER2; 1St. Michael\'s Hosp., Toronto, ON, Canada; 2Univ. of Toronto, Toronto, ON, Canada; 3Research, Physical Sci., Sunnybrook Hlth. Sci. Ctr., Toronto, ON, Canada
Wednesday, November 15, 2023 at 8:00AM-12:00PM